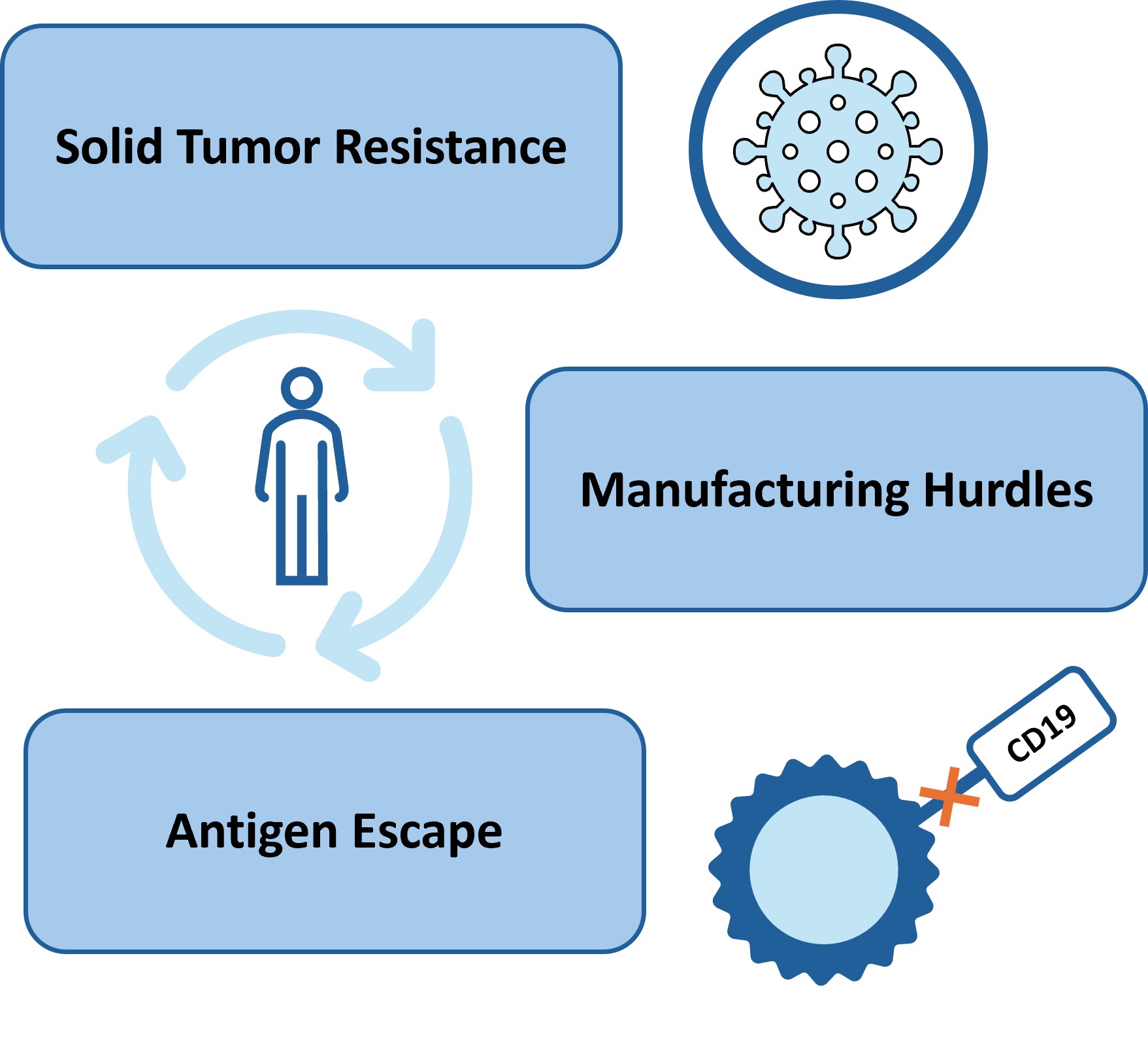
CAR-T cell therapy is revolutionary in cancer treatment. While producing many remarkable clinical results, many challenges limit the therapeutic efficacy of CAR-T cells in solid tumors and hematological malignancies. Challenges to effective CAR-T cell therapy include antigen escape, limited tumor infiltration and others.
CAR-T cell therapy also has manufacturing challenges that include high cost of production and long lead times contributing to delays in administering the therapies.
Luminary has developed a platform coupled with a novel set of CARs that address these challenges head on for both hematological malignancy and sold tumors.
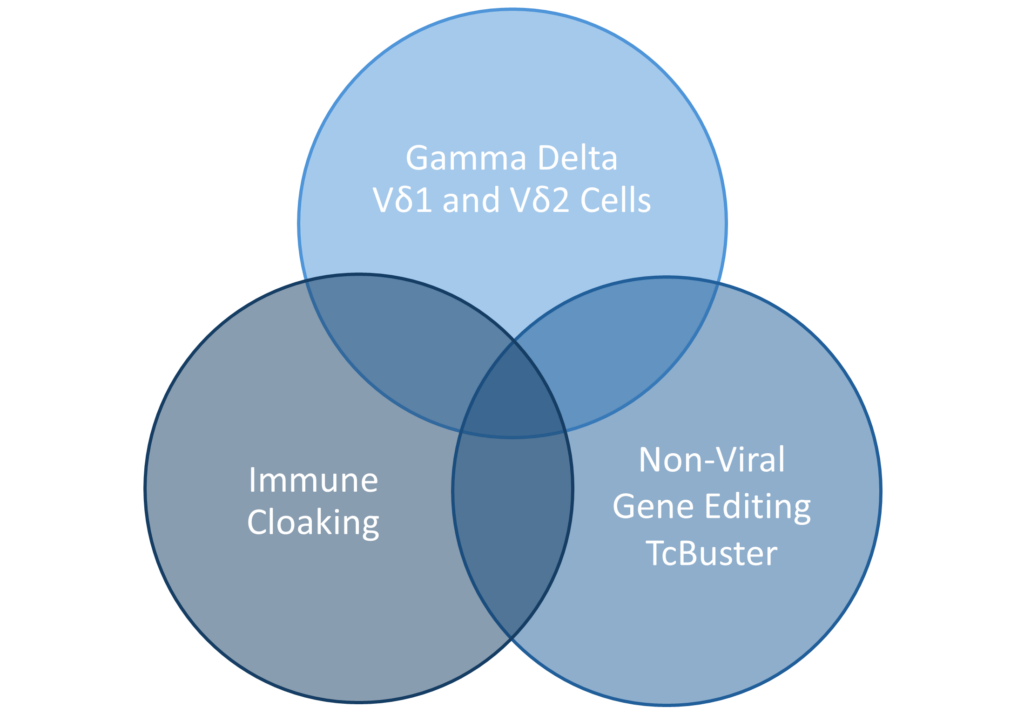
At Luminary, we are keenly focused on addressing the many challenges facing CAR T therapies. We developed a unique allogenic platform unlike any other. We are utilizing that platform to develop cellular therapies that bring new advances to the table. Our work is helping to deliver new standards of care for B-Cell malignancies and specific B-Cell mediated autoimmune diseases. At the same time, we are delivering ground-breaking science for solid tumors. And we are doing this at lower therapy costs.
Less relapse, more recovery, less financial strain. This is our mission.
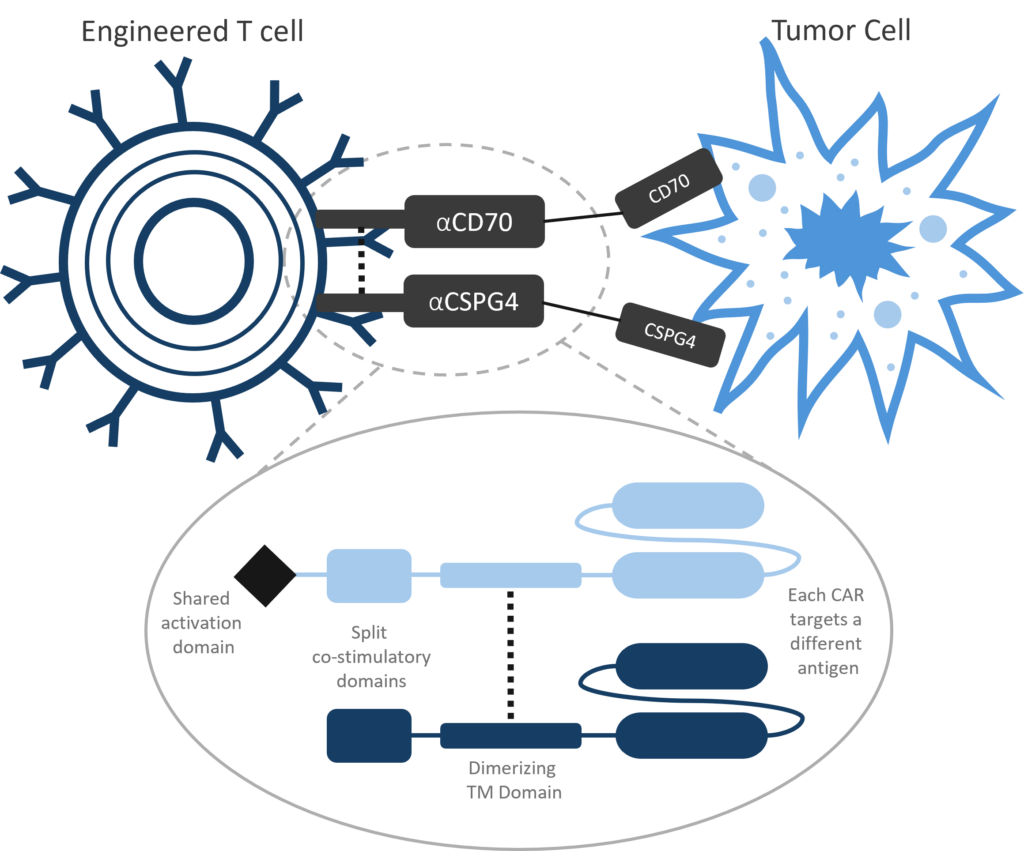
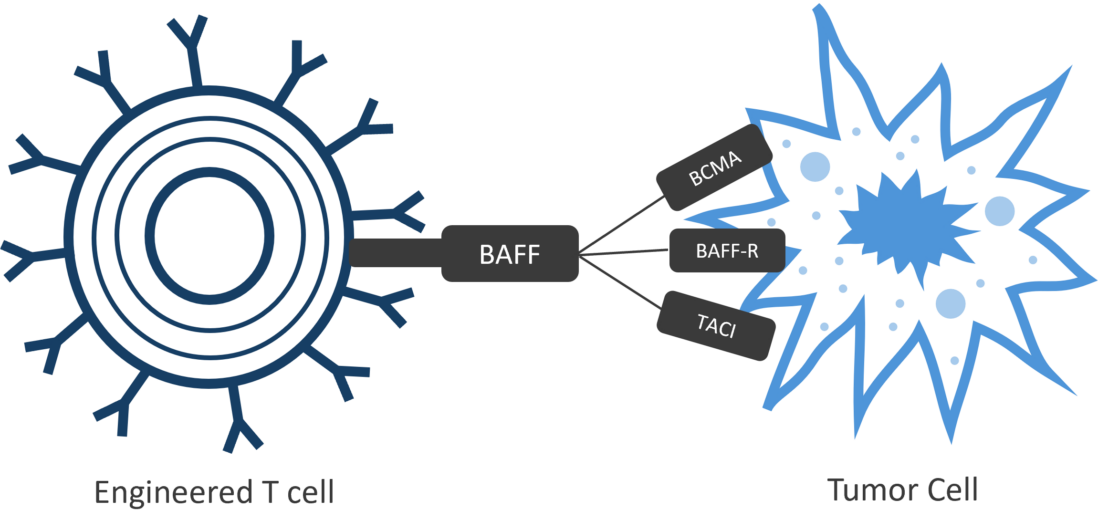
Luminary Therapeutics is combining CAR T design elements in novel ways to overcome the unique challenges presented by the solid tumor microenvironment. Our solid tumor program utilizes a Dual CAR architecture to target multiple cancer antigens and more completely eradicate cancerous cells with a single engineered T cell product. We have optimized our costimulatory domain arrangement to prevent T cell exhaustion and enhance persistence. Finally, the large genetic cargo capacity of the TcBuster transposon system allows us to include cytokine sinks to neutralize immunosuppressive signals in the tumor microenvironment.
Research faces a conundrum. While the use of CAR T-cell therapy has expanded for patients, relapse rates remain high. A recent study found 57% of all patients relapsed within a year of treatment. According to Liang et al Journal of Hematology & Oncology, CD-19 specific CAR T cell therapy has significantly improved the outcome of patients with relapse/refractory diffuse large B-Cell lymphoma (R/R DLBCL), resulting in durable remissions in approximately 40% of heavily pre-treated patients. Despite these encouraging results, nearly half of the patients could not achieve durable responses after CD19-CAR T therapy, and a significant proportion of patients will eventually relapse.
Clearly, as data from a larger patient population is published, a high risk of relapse has again emerged as a formidable obstacle on the road to long-term survival.
The need for effective therapies to lessen relapse—along with the need for rapid and cost-effective manufacturing—are among the most pressing matters we face. It is not an overstatement to say we’re in need of cell therapy that ultimately doesn’t lead to more treatment.
Copyright © 2019–2026 All Rights Reserved by Luminary Therapeutics.